The United States Food and Drug Administration finalized a regulation in early March 2023 that updates the reporting requirements for mammograms. The new regulations will come into effect on September 10, 2024 and will require all women to receive information about breast density after a mammogram. They will also need to be told in their mammogram report that dense breast tissue can mask cancer and make cancer harder to detect.
The Conversation asked Dr. Wendie A. Berg, professor of radiology at the University of Pittsburgh School of Medicine, to explain how the rule change could affect screening recommendations as well as how people interpret their results .
What is breast density and why is it important?
All breasts are made up of a mixture of fat, mammary glands and ducts. The glands are supported by fibrous tissue and ligaments, collectively called “fibroglandular tissue”. The more fibroglandular tissue a woman has, the “dense” her breast tissue is.
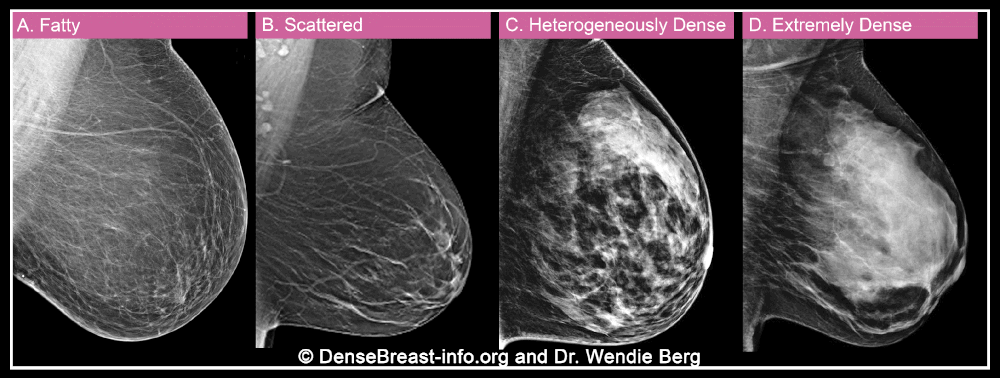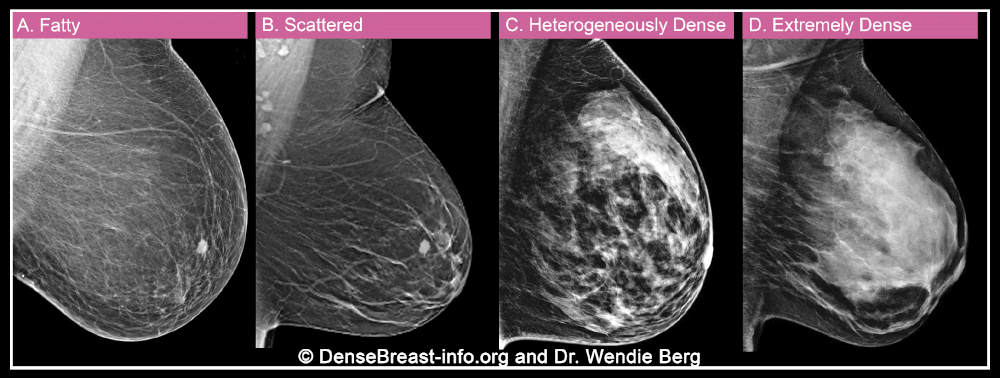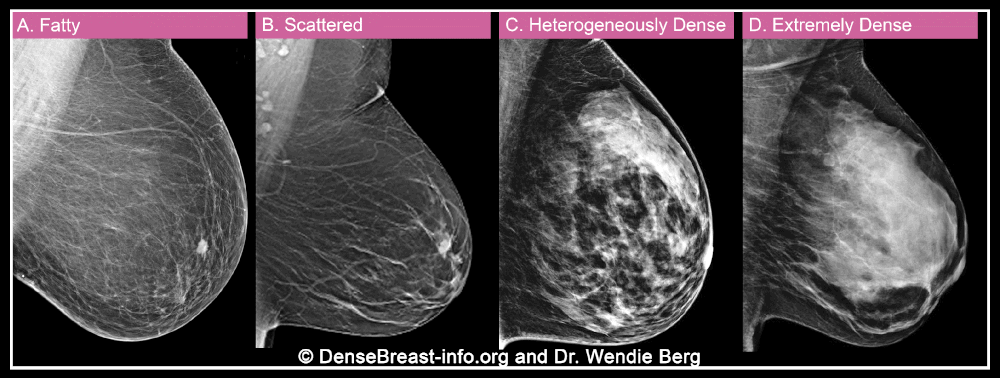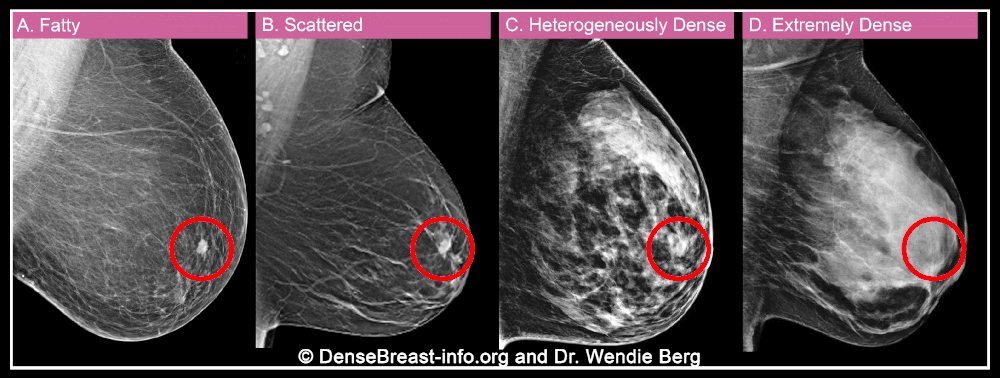
When a woman has a mammogram, the radiologist examining her will describe her breast density using one of four categories: A) fatty, B) sparse tissue, C) heterogeneously dense, or D) extremely dense. Categories C and D are considered “dense” while categories A and B are “non-dense”.
Dense breasts are normal and common. More than 50% of women have dense breasts before menopause, as do about 40% of women in their 50s and 30% of women in their 60s. Breasts may become less dense after menopause, but a woman with extremely dense breasts will likely continue to have dense breasts throughout her life.
Breast density is important for two reasons. More importantly, dense breast tissue can hide cancer on a mammogram. About 40% of breast cancers will go undetected on mammography in the densest breasts, labeled “extremely dense breasts,” and about 25% will go undetected in heterogeneously dense breasts.
Second, dense tissue also increases the risk of developing breast cancer, with about four times the risk of breast cancer in extremely dense breasts compared to fatty breasts, and about twice the risk compared to breasts with soft tissue. scattered.

DenseBreast-info.org and Dr. Wendie Berg
What does the FDA decision imply?
Until now, 38 states plus Washington, DC had different laws about what to tell women about breast density. This has resulted in inconsistent levels of information provided to American women based on where they live.
Beginning in September 2024, the FDA’s final rule creates a uniform national standard requiring all women to be told in the mammogram results letter that their breasts are either “dense” or “non-dense.” They will be told that dense tissue can hide cancer on a mammogram and also increases the risk of developing breast cancer.
The new regulation requires that specific density category be included in all mammogram reports that are sent to the referring health care provider. Some states require that the specific density category also be included in the patient’s results letter, and this information may be included, but should be separate from language required by the FDA. The FDA notification cannot be modified in any way.
The FDA requirement also includes this sentence in the letter to women with “dense” breasts: “In some people with dense tissue, other imaging tests in addition to a mammogram may help find cancers. Such “additional screening” deserves discussion.
How might this affect how patients react to mammogram results?
Without some guidance on what to do about it, this information may cause confusion and concern.
3D mammograms, also known as tomosynthesis, are becoming the norm and are slightly better at detecting cancer, with fewer callbacks for further testing for results that turn out not to be cancer. Women with dense breasts should be sure to have routine screening with a 3D mammogram.
The decision to pursue additional screening beyond an annual mammogram beginning at age 40 depends on several considerations. These include breast density and other risk factors, potential benefits, disadvantages – such as further testing for results that turn out not to be cancer – insurance coverage and costs.
At age 30, all women should discuss their risk factors with their health care provider and consider genetic testing if appropriate. Indeed, women considered “high risk” should begin screening earlier and undergo MRI screening in addition to mammography, regardless of breast density.
Here is a list of some of the factors that would make a woman “high risk” and good candidates for annual MRI screening until age 70-75, depending on general health.
-
Women with pathogenic genetic variants, such as BRCA1 or BRCA2, or who have a mother, sister, or daughter with a pathogenic variant, should begin annual MRI screening around age 25 to 30, and add mammography screening once they turn 30.
-
Women who received radiation therapy to the chest for a previous cancer – usually Hodgkin’s lymphoma – before age 30 should start MRI screening eight years after treatment – but not before age 25 – and add mammogram at age 30.
-
Women with an estimated lifetime risk of breast cancer of at least 20% should have an annual MRI, in addition to mammography. The most accurate estimates come from the Tyrer-Cuzick or IBIS model and include weight, height, breast density, family history, biopsy history, and other risk factors. AI-based processing of mammograms alone may be even more accurate than risk models in predicting who will develop breast cancer in the next one to five years.
-
Annual MRI screenings are also recommended for women diagnosed with breast cancer before age 50 or women with dense breasts.
-
The European Society for Breast Imaging recommends women with extremely dense breasts add MRI screening every 2-4 years from age 50-70 (with mammograms every 2 years).
Women with dense breasts, especially if they also have other risk factors such as a family history of breast cancer or a previous atypical biopsy, should consider adding screening MRI to their annual mammogram. But the MRI requires lying down in the magnet tunnel, which can be difficult for women with claustrophobia. It also requires an intravenous injection of contrast product. Cancers become more visible with contrast because they have more and more permeable blood vessels than normal tissue.
For women who cannot tolerate or access MRI, the addition of ultrasound to mammography can be considered, but MRI detects more cancers than ultrasound. Contrast mammography is being evaluated as an alternative to MRI.
Will insurance cover additional screening tests?
Currently, 15 states plus DC have laws requiring insurance coverage for supplemental breast cancer screening, but only New York, Connecticut, and Illinois require such coverage without a copay.
A federal insurance bill, the Find It Early Act, is reintroduced by two US representatives. This measure would ensure that all health insurance plans cover breast screening and diagnostic imaging at no cost.
This would include additional screening for women with dense breasts or at higher risk for breast cancer, according to National Comprehensive Cancer Network guidelines and American College of Radiology suitability criteria.
